A professional industrial design company specializing in medical devices will adhere to stringent quality control regulations in medical device design and product development processes. This is crucial for ensuring the efficiency and quality of medical product design and development. Here, we share the common steps in medical device product design and development.
1. Project Initiation and Approval
Normally when an industrial design company receives the medical product design requirements of a client, it issues a project task document announcing that henceforward the start of this project means work into designing and development began.

2. Planning for Medical Device Design and Development
After initiating the project, designers create a corresponding elite team based on specific design requirements, with each member responsible for their respective task. Once the team has completed designing and developing a concept, they propose different concepts such as the function of the medical device meaning what it does appearance of structural integrity , materials used in its manufacturing process
3. Key Inputs in Design and Development
The product’s functions, features, safety requirements and risk management regulations are defined based on findings of market research. This involves considering various aspects: those of the product’s principal application, characteristics and benefits , potential in service to satisfy such needs as personnel workload equipment air temperature safety precautions stability of position time span available raw materials etc. These aspects are critically reviewed, confirmed and documented with rigorous precision before necessary documentation is circulated.
4. Outputs in Design and Development
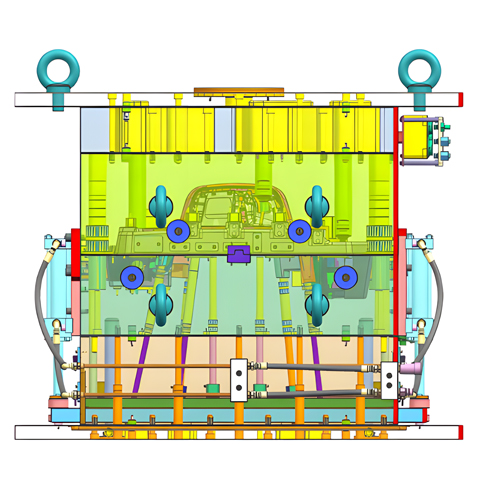
The design output should first meet the design input requirements. It must specify the required raw materials, components, technical standards, acceptance rules, product execution standards, engineering drawings, component details, production process flow, machining process, production line equipment, prototypes, testing procedures and methods, packaging, and packaging labels in detail. Additionally, one should meticulously maintain records of the design and development process.

5. Review of Design and Development Plans
One should systematically review design and development plans to ensure their applicability and effectiveness. This process verifies that the outcomes meet the overall objectives. The purpose is to evaluate whether the results of the design and development phases meet the specified requirements of the design plan and comply with relevant laws and regulations. This step identifies any shortcomings and proposes solutions to prevent product non-conformity at an early stage.

6. Verification of Design and Development
To ensure the outputs of medical device product design and development meet the specified inputs, one should conduct verification according to the planned allocation. Verification methods include: using different approaches to design and verify against the design data or requirements; comparing with similar designs; prototyping for testing and demonstration; self-testing of prototypes; third-party testing; and document review.
7. Validation of Design and Development
To ensure that the product meets the required usability standards or the known anticipated primary use, validation of the medical device product’s design and development should be conducted according to the design plan’s allocation. This includes clinical evaluation, simulation and comparison reviews, and feature evaluations.
Additionally, one should conduct design changes and change reviews if necessary. It’s essential to clearly state the reasons, requirements, and standards for changes, and these changes must also undergo review.